Characteristics of the study population
Between May 12, 2021 and October 31, 2021, within a population of 32 million persons aged 12 to 50 years, 21.2 million first (19.3 million second) doses of the BNT162b2 vaccine and 2.86 million first (2.58 million second) doses of the mRNA-1273 vaccine were received (Table S1). In the same period, 1612 cases of myocarditis (of which 87 [5.4%] had also a pericarditis as associated diagnosis) and 1613 cases of pericarditis (37 [2.3%] with myocarditis as associated diagnosis) were recorded in France. We matched those cases to 16,120 and 16,130 control subjects, respectively. The characteristics of the cases and their matched controls are shown in Table 1. For both myocarditis and pericarditis, key differences between cases and controls included a higher proportion among cases of a history of myocarditis or pericarditis, of history of SARS-CoV-2 infection, and receipt of an mRNA Covid-19 vaccine. The mean age and proportion of women were lower among patients with myocarditis than those with pericarditis.
Risk of myocarditis and pericarditis associated with vaccination
For both vaccines, the risk of myocarditis was increased in the seven days post vaccination (Table 2; in the rest of the text, we will refer to multivariable odds ratios). For the BNT162b2 vaccine, odds ratios were 1.8 (95% confidence interval [CI]: 1.3–2.5) for the first dose and 8.1 (95% CI, 6.7–9.9) for the second. The association was stronger for the mRNA-1273 vaccine with odds-ratios of 3.0 (95% CI, 1.4–6.2) for the first dose and 30 (95% CI, 21–43) for the second. The risk of pericarditis was increased in the seven days following the second dose of both vaccines, with odds ratios of 2.9 (95% CI, 2.3–3.8) for the BNT162b2 vaccine and 5.5 (95% CI, 3.3–9.0) for the mRNA-1273 vaccine. Vaccination in the previous 8 to 21 days, with either the BNT162b2 or mRNA-1273 vaccine was not associated with a risk of myocarditis or pericarditis. Independently of vaccination status, a history of myocarditis was strongly associated with a risk of contracting myocarditis during the study period, with an odds-ratios of 160 (95% CI, 83–330). The same was true for pericarditis, with an odds ratio of 250 (95% CI, 120–540). No interaction was found between history of myocarditis or pericarditis and vaccine exposure. Infection with SARS-CoV-2 in the preceding month was also associated with a risk of myocarditis (odds ratio, 9.0 [95% CI, 6.4–13]) or pericarditis (odds ratio, 4.0 [95% CI, 2.7–5.9]).
Subgroup estimates by sex and age classes
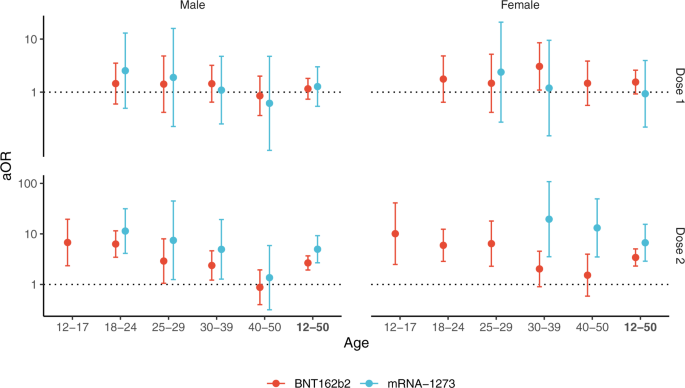
The risk of myocarditis was substantially increased within the first week post vaccination in both males and females (Fig. 1 and Table S2). Odds-ratios associated with the second dose of the mRNA-1273 vaccine were consistently the highest, with values up to 44 (95% CI, 22–88) and 41 (95% CI, 12–140), respectively in males and females aged 18 to 24 years but remaining high in older age groups. Odds-ratios for the second dose of the BNT162b2 vaccine tended to decrease with age, from 18 (95% CI, 9–35) and 7.1 (95% CI, 1.5–33), respectively in males and females aged 12 to 17 years, down to 3.0 (95% CI, 1.5–5.9) and 1.9 (95% CI, 0.39–9.3), respectively in males and females aged 40 to 51 years.
Adjusted odds-ratio (aOR) from multivariable model are represented in base 10 logarithmic scale according to age groups (x-axis), by sex (columns) and vaccine dose ranking (rows). Colors denote the type of vaccine. Centre value are aOR point estimates and error bars represent 95% confidence intervals. Number of cases (N) by age categories (12–17, 18–24, 25–29, 30–39, 40–50 and 12–50 years) are respectively as follows: N = 137, 480, 210, 273, 181 and 1281 for males, and N = 29, 106, 40, 88, 68 and 331 for females. aOR could not be calculated in categories where no case exposed to vaccine was recorded, for instance for males and females aged 12 to 17 years having received the mRNA-1273 vaccine.
An increased risk of pericarditis was also found in the first week after the second dose of either of the mRNA vaccines among both males and females (Fig. 2 and Table S3). Odds-ratios for the second dose of the BNT162b2 vaccine showed a downward trend across age groups with values up to 6.8 (95% CI, 2.3–20) and 10 (95% CI, 2.5–41), respectively in males and females aged 12 to 17 years. The second dose of the mRNA-1273 vaccine was associated with pericarditis among males and among females only within age 30 to 39 years (odds-ratio 20 [95% CI, 3.5–110]) and age 40 to 50 years (odds-ratio 13 [95% CI, 3.5–49]).
Adjusted odds-ratio (aOR) from multivariable model are represented in base 10 logarithmic scale according to age groups (x-axis), by sex (columns) and vaccine dose ranking (rows). Colors denote the type of vaccine. Centre value are aOR point estimates and error bars represent 95% confidence intervals. Number of cases (N) by age categories (12–17, 18–24, 25–29, 30–39, 40–50 and 12–50 years) are respectively as follows: N = 65, 194, 106, 282, 342 and 989 for males, and N = 36, 118, 91, 183, 196 and 624 for females. aOR could not be calculated in categories where no case exposed to vaccine was recorded, for instance for males and females aged 12 to 17 years having received the mRNA-1273 vaccine.
Associations between vaccination within the seven preceding days and the risk of myocarditis or pericarditis were of the same magnitude when the analysis was restricted to the period prior to the warning against myocarditis and pericarditis as adverse events sent to prescribers on July 19, 2021 (Fig. S1 and Table S4). The results were unchanged in models excluding patients with a history of SARS-CoV-2 infection in the past month, those with a history of myocarditis or pericarditis within five years, those diagnosed with both myocarditis and pericarditis, or those with a hospitalization within a month prior to index date.
Excess events
We estimated the number of excess cases attributable to vaccines by sex and age group (Fig. 3). The number of excess cases of myocarditis per 100,000 doses administered to adolescent males 12 to 17 years was 1.9 (95% CI, 1.4–2.6) for the second dose of the BNT162b2 vaccine and for young adults 18 to 24 years of age reached 4.7 (95% CI, 3.8–5.8) for the second dose of the BNT162b2 vaccine, and 17 (95% CI, 13–23) for the second dose of the mRNA-1273 vaccine (Fig. 3). This translates into one case of vaccine-associated myocarditis per 52,300 (95% CI, 38,200–74,100) second doses of the BNT162b2 vaccine among 12–17 years, and 21,100 (95% CI, 17,400–26,000) second doses of the BNT162b2 vaccine and 5900 (95% CI, 4400–8000) second doses of the mRNA-1273 vaccine among 18–24 years (Table S5). Estimates of excess cases were lower for older age groups and generally for females. However, the number of excess cases of myocarditis attributable to the second dose of the mRNA-1273 vaccine was consistently higher. Among females aged 18 to 24 years, the estimated number of excess cases of myocarditis per 100,000 doses reached 0.63 (95% CI, 0.34–1.1) for the second dose of the BNT162b2 vaccine (corresponding to 1 case per 159,000 [95% CI, 90,800–294,400] doses) and 5.3 (95% CI, 3.0–9.1) for the second dose of the mRNA-1273 vaccine (corresponding to 1 case per 18,700 [95% CI, 11,000–33,400] doses). The number of excess cases of pericarditis is presented in Fig. 3. As for myocarditis, estimates for the second dose of the mRNA-1273 vaccine were consistently higher.
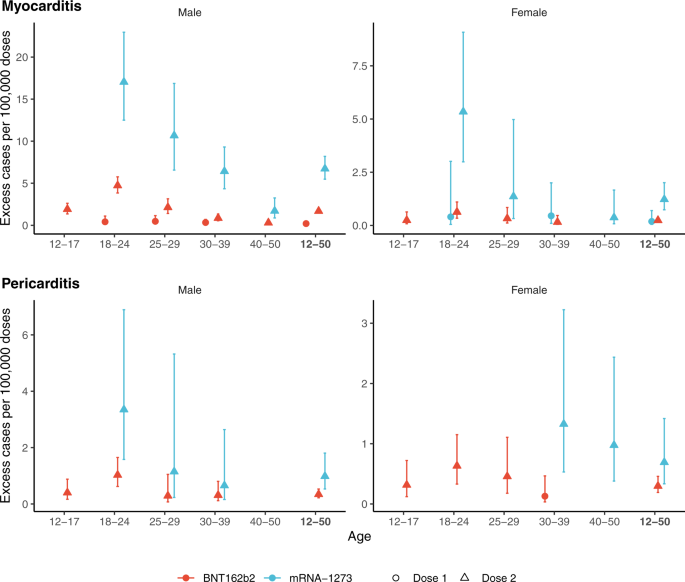
Excess cases are based on the risk in the 7 days following vaccination. Colors denote the type of vaccine and the shape of point estimate denotes the ranking of dose vaccine. Centre value are excess cases point estimates and error bars represent 95% confidence intervals. Number of cases (N) by age categories (12–17, 18–24, 25–29, 30–39, 40–50 and 12–50 years) are respectively as follows: for cases of myocarditis, N = 137, 480, 210, 273, 181 and 1281 in males, and N = 29, 106, 40, 88, 68 and 331 in females; for cases of pericarditis, N = 65, 194, 106, 282, 342 and 989 in males, and N = 36, 118, 91, 183, 196 and 624 in females. Excess cases was only calculated in categories with a significantly positive association between the vaccine exposure and the outcome (adjusted odds-ratio >1).
Characteristics of myocarditis and pericarditis cases occurring after vaccination
Among exposed cases, the delay between administration of the vaccine and hospitalization (Fig. S2) was shorter after the second dose than after the first dose, both for myocarditis (median of 4 days versus 10 days after the BNT162b2 vaccine and of 3.5 days versus 9 days after the mRNA-1273 vaccine) and for pericarditis (median of 6 days versus 10 days after the BNT162b2 vaccine and of 3 days versus 11 days after the mRNA-1273 vaccine).
Table 3 shows the characteristics of cases acquired within 7 days of vaccination (deemed post-vaccination cases) compared to those acquired within a larger delay or in the absence of vaccination. Post-vaccination cases were significantly younger (predominantly in 18 to 24 years), more frequently concerned males for myocarditis but not for pericarditis, and without a history of myocarditis or pericarditis, respectively, or of SARS-CoV-2 infection. The lengths of hospital stay were not significantly different in post-vaccination cases of myocarditis (median 4 days) and pericarditis (median 2 days) than in unexposed cases. The frequency of admission in intensive care unit, mechanical ventilation or death was lower for post-vaccination cases than for unexposed cases. After a follow-up of 30 days after discharge, 4 (0.24%) deaths among cases of myocarditis (none among exposed to vaccine) and 5 (0.31%) deaths among cases of pericarditis (including one patient having received a vaccine 8 to 21 days prior to the diagnosis) were reported. Of those, 3 and 2 died during their hospital stay for myocarditis and pericarditis, respectively.
Drugs treatments within 30 days after hospital discharge are presented in Figs. S3 and S4. Regardless of the vaccination status, the therapeutic classes most frequently used during the follow-up of myocarditis cases included beta blocking agents (63% of patients), analgesics (52%) and agents acting on the renin−angiotensin system (46%). The corresponding treatments of pericarditis cases were analgesics (83%), colchicine (69%) and beta blocking agents (14%) (Fig. S4).