This ribbon diagram shows the structure of the Delta variant’s spike protein before the virus fuses with its target cell. The N-terminal domain (NTD) is shown in blue and the receptor-binding domain (RBD) in cyan. Credit: Bing Chen, PhD, Boston Children’s Hospital
Findings have implications for next-generation
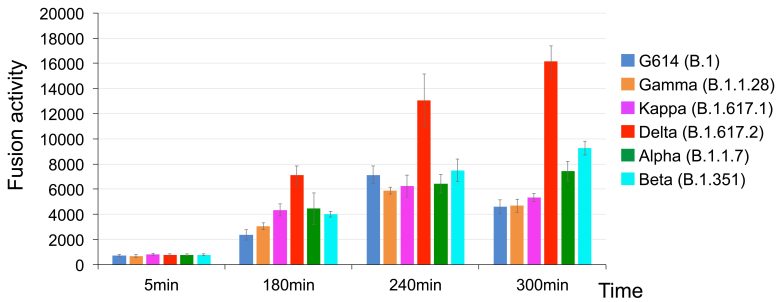
The Delta variant of SARS-CoV-2 fused to cell membranes far more rapidly than five other variants (Alpha, Beta, G614, Gamma, and Kappa). Credit: Zhang J; et al. Science 2021 Oct 26; DOI: 10.1126/science.abl9463
“Membrane fusion requires a lot of energy and needs a catalyst,” explains Chen. “Among the different variants, Delta stood out in its ability to catalyze membrane fusion. This explains why Delta is transmitted much faster, why you can get it after a shorter exposure, and why it can infect more cells and produce such high viral loads in the body.”
Designing interventions, informed by structure
To learn how mutations in the variants affect the spike protein’s structure, Chen and colleagues used cryo-electron microscopy, which has resolution down to the atomic level. They imaged spike proteins from the Delta, Kappa, and Gamma variants, and compared them to spikes from the previously characterized G614, Alpha, and Beta variants.
All the variants had changes in two key parts of the spike protein that are recognized by our immune system’s neutralizing antibodies: the receptor-binding domain (RBD), which binds to the ACE2 receptor, and the N-terminal domain (NTD). Mutations in either domain can make neutralizing antibodies less able to bind to the spike.
“The first thing we noticed about Delta was that there was a large change in the NTD, which is responsible for its resistance to neutralizing antibodies,” Chen says. “The RBD also changed, but this led to little change in antibody resistance. Delta still remained sensitive to all the RBD-targeted antibodies that we tested.”
Looking at the other variants, the researchers found that each modified the NTD in different ways that altered its contours. The RBD was also mutated, but the changes were more limited. Overall, the RBD’s structure remained relatively stable across the variants, likely to preserve its crucial role in binding to the ACE2 receptor. The researchers therefore believe that the RBD is a more favorable target for the next generation of vaccines and antibody treatments.
“We wouldn’t want to target the NTD, because the virus can quickly mutate and change its structure; it’s a moving target,” elaborates Chen. “It might be most effective to target the RBD — to focus the immune system on that critical domain rather than the whole spike protein.”
Reference: “Membrane fusion and immune evasion by the spike protein of SARS-CoV-2 Delta variant” by Jun Zhang, Tianshu Xiao, Yongfei Cai, Christy L. Lavine, Hanqin Peng, Haisun Zhu, Krishna Anand, Pei Tong, Avneesh Gautam, Megan L. Mayer, Richard M. Walsh, Jr., Sophia Rits-Volloch, Duane R. Wesemann, Wei Yang, Michael S. Seaman, Jianming Lu and Bing Chen, 26 October 2021, Science.
DOI: 10.1126/science.abl9463
Jun Zhang, PhD, and Tianshu Xiao, PhD, of Boston Children’s Hospital were co-first authors on the paper. The study was funded by Emergent Ventures, the Massachusetts Consortium on Pathogen Readiness (MassCPR), and the National Institutes of Health (grants AI147884, AI141002, AI127193, AI39538, and AI165072).